Adenomyosis
Adenomyosis is the main pathology in
this subgroup. It is diagnosed when benign endometrial glands and stroma invade
2-3 mm into the uterine muscle (1-2 high power fields below the endometrium).
This is usually followed by hyperplasia and hypertrophy of the adjacent muscles
due to repeated bleeding episodes during menstruation. It is more common
in women over the age of 40 years who might account for 80% of all cases.
However, it is also more associated with the following:
- Previous repeated childbirth as 80% of all cases are parous women;
- After intrauterine operations including D&C and evacuation of the uterus;
- Some reports associate adenomyosis with previous caesarean section and tubal ligation.
A common
factor in most of these causes is the breakdown in the normal endometrial /
myometrial interface which can allow migration of endometrial glands and
stroma into the adjacent muscle. There is 80% chance of an associated
other pelvic pathology, mainly fibroids in 50%, endometriosis in 11% and
endometrial polyps in 7% of the cases. The association with endometriosis was
further elucidated by a recent study conducted by
Kunz et al [2007]. Using MRI studies, they showed that the dorsal
junctional zone of the uterus had already been invaded by the basal endometrium
by the third decade of life in women with endometriosis. No such invasion was
seen in women with no endometriosis up to the age of 34 years.
Presentation
- Pelvic
pain
- painful
menstruation
- Heavy
periods
- Painful
intercourse
- Infertility
However, in almost 50% of the cases there are no related symptoms and it
is only a chance finding. It is more likely that symptoms occur with deeper
penetration and wider distribution of the pathology.
Distribution of adenomyosis
There are two types of
adenomyosis, diffuse and focal types. Diffuse adenomyosis occupies a wider area of the uterine muscle and may show
as:
- Asymmetrical
areas of altered myometrial echogenicity on transvaginal scan examination.
These may show as multiple stripes down the picture.
- Hypoechoic
cysts or focal areas with hypo or hyperechogenic texture.
- Colour
Doppler may show normal blood vessels distribution in small focal
subendometrial lesions. However, a diffuse pattern can be seen with
extensive lesions, unlike the basket pattern characterised by peripheral
blood vessels usually seen with fibroids. This is best
demonstrated immediately after injecting a contrast medium. However, Doppler
studies are not usually necessary to help with the diagnosis of adenomyosis.
On
the other hand focal adenomyosis may be localised to one or two areas mostly
subendometrial. Alternatively, it may show as an indiscriminate endometrial / myometrial interface, causing difficulty in clear identification of the junctional during transvaginal scan examination.
Other differences may also exist in relation to the histological
features, growth pattern, and response to ovarian hormonal changes and response
to different treatment modalities, as reported by Tamai et al in 2005. This may explain the differences in
presentation mode and response to treatment shown by different patients with
the same degree of the disease.
Diagnosis
of adenomyosis
The uterus
is usually enlarged but regular in shape. It may be tender during bimanual
vaginal examination. Previously histological examination of hysterectomy
specimens was the only means for diagnosing adenomyosis. However, recently both
MRI and transvaginal scan examinations proved useful for the pre-surgical
diagnosis of the disease. MRI is very sensitive in showing widening of the
junctional zone but transvaginal scan examination can also be 80% sensitive
in diagnosing adenomyosis. In a recent study, Kepkep et al (2007) examined
the most reliable ultrasonic uterine findings in women with histologically
confirmed adenomyosis. They found that regular enlargement of the uterus with a
globular appearance and myometrial cysts were very accurate in diagnosing
adenomyosis. However, the highest accuracy was shown by the presence of
subendometrial linear striations. Interestingly, the same study showed a
stronger role for transvaginal scan examination in excluding adenomyosis than
confirming it. The negative and positive diagnostic values were 84.4% and 55.3%
respectively. This might have ben a reflection of the small number of patients
examined. Then again, transvaginal scanning may facilitate needle biopsy and
histological examination of suspected areas. On the other hand,
hysterosalpingography is not very sensitive and may show 1-4 mm spikes into
the myometrium.
Adenomyotic glands are not fully responsive to progesterone yet transvaginal scan examinations during the luteal phase are more likely to give a better illustration of subendometrial focal lesions as shown by Abdel-Gadir et al in 2012. The echogenic endometrium will stand out against the hypoechoic myometrial cystic shadows. Accordingly, a better picture can be obtained by timing examinations during the late luteal phase in suspected cases.
- The first ultrasound image above shows an axial view of an enlarged uterus with nonohomogenous myometrium and indescriminate endometrial echo.
- The second image shows the same uterus during the luteal phase. Note the appearance of two hypoechoic cystic area which facilitated the diagnosis of adenomyosis.
- The third image shows a wide posterior uterine wall compared to the anterior one, with the endometrial line in between. Note the non homogeneous posterior wall myometrium, and the indiscriminate endometrial echo on the left side of the cavity.
In some cases, 3D ultrasonography is superior to 2D imaging and may be the only way for diagnosing adenomyosis in suspected cases. This is especially so in cases with fundal lesions as shown by the two ultrasound images below.
- The first image is a full multiplanar view of a uterus. The sagittal and axial views showed no evidence of any areas suggestive of adenomyosis. However the 4th enclosed images showed few phalanges extending out of the fundal area.
- The second image is a 3D view of the same uterus magnified to show the extent of extension of the adenomyotic projections into the fundal area of the uterus.
Doppler studies for adenomyosis
In some cases, the use of Doppler colour mapping may help in confirming the diagnosis of adenomyosis, especially when 2D ultrasonography is not very affirmative. It is also useful to differentiate it from fibroids.
- The first colour Doppler image above shows a cluster of vessels passing into a circumscribed adenomyotic area on the posterior fundal wall of a retroverted uterus. The posterior endometrial / myometrial junctional zone was not involved. This was made clear by the trilaminar endometrium in front.
- The second image shows an anteverted uterus with a wide non-homogenous area in front and behind the inconspicuous early follicular endometrial line. Also note the through vascular flow pattern into the adenomyotic area in the front wall. Compare this to the pattern seen in fibroids as depicted below. This patient had menorrhagia, very bad dysmenorrhoea and dyspareunia. The extent of the uterine lesions were reflected by her very intense symptoms.
For comparison purpose, see the two colour Doppler images below. They show peripheral distribution of blood vessels around different fibroids. This is very different to the through patten frequently seen in some adenomyotic lesions
Role of hysteroscopy in diagnosing adenomyosis
Generally speaking, hysteroscopy is not very useful as a primary technique for establishing the diagnosis in most
cases. A hysteroscopically directed single biopsy is less than 20% sensitive in
diagnosing adenomyosis. However, it is 100% specific when the correct area has
been biopsied. This low sensitivity may be improved by using transvaginal scan
directed biopsies. Deep diffuse lesions are not accessible hysteroscopically, but subendometrial lesions can occasionally show in different ways, mainly as bruised circumscribed areas. Reducing
intrauterine pressure during endometrial resection may allow the adenomyotic
lesions to hang down as dark or black projections into the uterine cavity after
excision of the superficial area overlying the lesions.
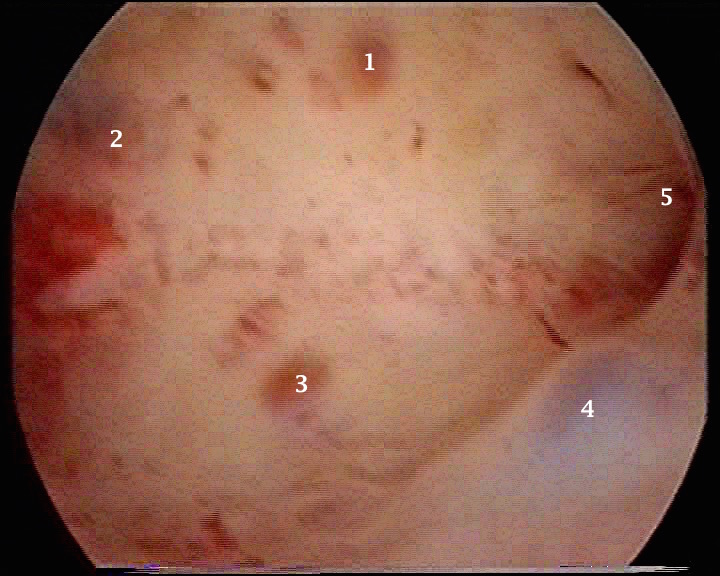 |  |
- The first hysteroscopic image above shows landmarks within the cavity. Points 1 - 4 depict adenomyotic
lesions while 5 depicts the ostium of the left tube. Subendometrial adenomyotic lesions may show as bruised areas or haemorrhagic bullae.
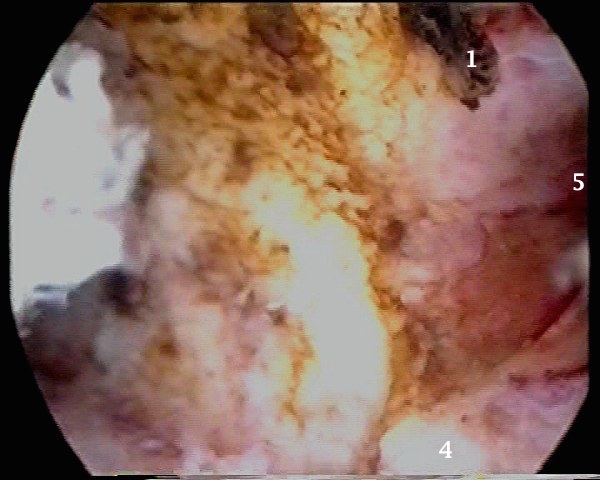
- The second image depicts the same uterus after excision of the superficial area over point 1 in the first image. It revealed dark adenomyotic tissue hanging into the cavity after reducing the
pressure during the procedure.
Management of adenomyosis
As up to 50% of patients with
adenomyosis are not symptomatic, treatment should not be offered on a
coincidental ultrasonic diagnosis. It should depend on the mode of
presentation whether it is pain, menorrhagia or infertility.
Medical
treatment is usually directed towards controlling pain and abnormal uterine
bleeding with:
· Nonsteroidal
anti-inflammatory drugs could be used for pain control but are not definitive
treatment of the disease. They could reduce menstrual blood loss as well.
However, their use could be restricted by gastrointestinal symptoms and changes
in platelets function. They could be used both for pain rescue and in a time
contingent protocol. Patients who wish to get pregnant
could use them to relieve menstrual pains.
· Prolonged
suppression with GnRH-analogues is currently the medical treatment of choice as
it suppresses ovulation and hence menstruation. However, it causes severe
hypo-oestrogenic side effects unless used with add-on medication. However, in
all cases symptoms do recur after suspending medication.
· The
levonorgestrel intrauterine device (mirena) has emerged as a very useful method
for controlling both adenomyosis symptoms namely pelvic pain and excessive
blood loss. However, its use should be
limited to those patients who are not keen to get pregnant.
It is evident that medical treatment can only offer symptomatic relief and recently uterine artery embolisation has been
used for the treatment of adenomyosis. Several studies reported improvement in
symptoms and quality of life with a decrease in uterine size and shrinkage of
the junctional zone thickness after embolisation [Siskin et al, 2001; and Kim et al, 2007]. A further advantage was the fact that
hysterectomy was avoided in the vast majority of patients [Lohle et al, 2007]. On the other hand, despite the good
success rates reported after uterine artery embolisation, laparoscopic uterine
arteries ligation did not give favourable results with adenomyosis [Wang et al, 2002]. This is in contradistinction with
fibroids where both techniques proved to be clinically successful.
Ultimately a conservative or more
likely a radical surgical intervention may be necessary. This is especially
so in cases of advanced adenomyosis causing uncontrollable pain and heavy
bleeding in women with no fertility aspiration. A review of the therapeutic options for the treatment of adenomyosis has
been published by Levgur M [2007].
· Selective
hysteroscopic resection after GnRH-a therapy may be helpful in patients with
up to 3 mm subendometrial focal lesions presenting with excessive bleeding.
However, it is only 50% successful in controlling pain. Both deep focal
and diffused lesions can be managed by laparoscopic electrocoagulation or
myometrial excision when future fertility is required. However, all these
measures are only temporary with a high risk of recurrence.
· On
the other hand hysterectomy can be offered as a primary procedure
to women who completed their families and the uterus is totally damaged by
extensive adenomyosis or multiple fibroids. It may as well be offered to
patienrts who failed to respond to medical or conservative surgical treatment.
Supra cervical hysterectomy is regaining popularity, when there is no cervical
pathology, as it entails no disturbace to the bladder bed or vaginal vault. It is an
easier procedure with less morbidity and quicker recovery time. The patient, however, needs to continue with her cervical screening tests as usual. Furthermore, up to 80% pain relief has
been suggested after a hysterectomy. This is because severe pelvic pain is usually
multifactorial and other causes may be present. This is especially so as 80% of patients may have both adenomyosis and pelvic endometriosis at the same time. Accordingly, exclusion of the presence of later may be advisable before proceeding with a hysterectomy, bearing in mind that 50% of women with isolated diagnosis of adenomyosis are symptomatic.
In our practice, we usually deal with any coexisting deep endometriosis, both surgically and medically before resorting to a more drastic hysterectomy. The presence of the uterus may sometimes help in preventing severe adhesions between the bladder anteriorly and the rectum and sigmoid posteriorly.



